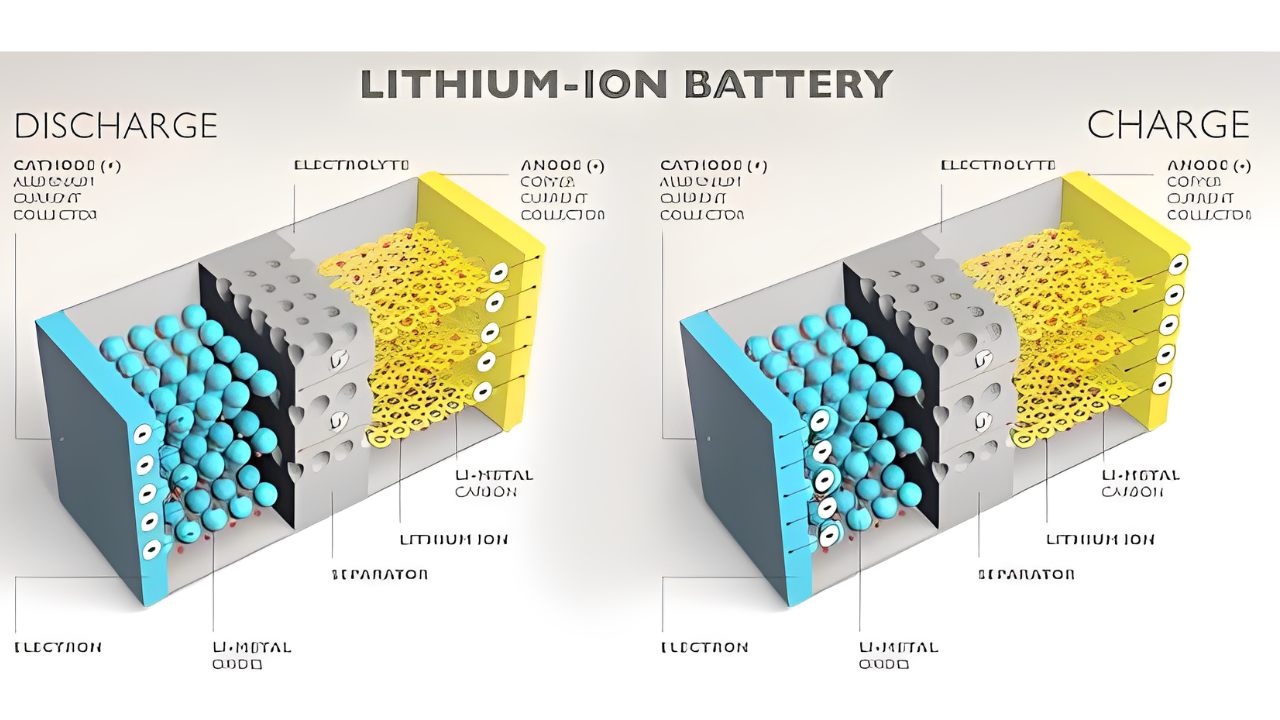
A lithium-ion battery works by using lithium ions to transfer between positive and negative electrodes in order to generate electrical energy. This type of battery is commonly used in portable electronic devices such as smartphones and laptops due to its high energy density and long lifespan.
Lithium-ion batteries have revolutionized the way we power our electronic portables. From mobile phones to laptops, these batteries are the driving force behind their seamless functionality.
But have you ever wondered how exactly a lithium-ion battery works? We will delve into the inner workings of these powerful batteries and explore the fundamental principles that allow them to store and discharge electrical energy efficiently.
By understanding the intricate mechanisms at play, you will gain a deeper appreciation for the technology that keeps your devices powered on the go. So, let’s unlock the secrets of the lithium-ion battery and unravel its captivating science.
The Basics Of Lithium Ion Batteries
Lithium ion batteries work by transferring lithium ions between two electrodes, typically made of lithium cobalt oxide and graphite. This movement of ions allows the battery to store and release energy, making it a popular choice for portable electronics and electric vehicles.
An Overview Of Battery Types
In the world of portable electronics, lithium-ion batteries are now the preferred power source for many different types of gadgets. From mobile phones and laptops to electric vehicles and power tools, these compact and lightweight batteries offer long-lasting energy storage with minimal maintenance required. But what sets lithium-ion batteries apart from other battery types?
The Composition Of A Lithium Ion Battery
The composition of a lithium-ion battery is critical to its efficient and reliable operation. These batteries consist of several essential components, each playing a vital role in the overall functioning of the battery.
First, we have the anode, which is typically made of carbon-based materials. This is where the lithium ions are stored when the battery is charged. On the other end, we have the cathode, which is typically made of a metal oxide compound. The cathode is in charge of holding and releasing the lithium ions while the device is charging or discharging process.
Between the anode and cathode, we have the electrolyte, which is a solvent that allows the movement of lithium ions between the two electrodes. Typically, the electrolyte is a liquid or gel-like material that contains lithium salts.
To keep everything in place and prevent short circuits, the battery also includes a separator, which physically separates the anode and cathode while allowing the flow of lithium ions.
How Lithium Ions Move
In a lithium-ion battery, lithium ions move from the anode to the cathode during discharge and from the cathode to the anode during charge. This movement is made possible by the flow of electrons through an external circuit.
When the battery is being supplied with external power source applies a voltage that drives a current through the battery.
This causes lithium ions to flow from the anode, where they are stored, to the cathode. Meanwhile, electrons flow through the external circuit, providing the energy needed to charge the device.
During discharge, the process is reversed. The stored lithium ions move from the anode to the cathode, releasing energy in the form of a flow of electrons. This flow of electrons powers the device, whether it’s a smartphone screen lighting up or an electric vehicle moving forward.
Overall, these are the basics of how a lithium-ion battery works. Understanding their composition and the movement of lithium ions allows us to appreciate the efficiency and versatility that these batteries bring to our everyday lives.

Credit: pubs.acs.org
The Process Of Charging
Understanding how a lithium-ion battery works involves grasping the intricate process of charging. The transfer of energy during charging is made possible through the combined efforts of the cathode, anode, and electrolyte. Let’s delve into each component’s role and how they contribute to the efficient functioning of these batteries.
The Role Of The Cathode And Anode
In a lithium-ion battery, the cathode and anode play crucial roles in the charging process. The cathode, typically made of a lithium-containing substance, such as an oxide of lithium, acts as the positive electrode.
When charging, the cathode absorbs lithium ions from the external power source. This absorption is reversible, allowing lithium ions to move back and forth between the cathode and the anode.
The anode, made of graphite or a similar material, functions as the negative electrode. As the external power source charges the battery, the anode releases electrons that flow through an external circuit, powering various devices.
From the anode to the cathode, electrons move during charging and from the cathode to the anode during discharging.
The Flow Of Electrons
The flow of electrons within a lithium-ion battery is a critical aspect of the charging process. When connected to a power source, such as a charger, the battery experiences an electrical potential difference between the cathode and the anode.
As a result, the free electrons within the anode start to move across the external circuit, creating an electric current that charges the battery.
This flow of electrons is made possible by the movement of lithium ions within the battery. As the battery, Lithium ions go from the cathode to the anode as charges through the electrolyte, which acts as a conductive medium.
Meanwhile, the movement of electrons between the cathode and anode completes the electrical circuit, enabling the battery to receive and store electrical energy.
The Role Of The Electrolyte
The electrolyte in a lithium-ion battery serves as a bridge between the cathode and the anode, facilitating the movement of lithium ions. Typically, the electrolyte is a liquid or gel-like substance that contains dissolved lithium salts.
This solution not only allows for a high conductivity but also prevents the electrodes from coming into direct contact, reducing the risk of short-circuiting.
Moreover, the electrolyte ensures the lithium ions move in a controlled manner during the charging process. It assists in preventing the buildup of undesirable chemical reactions within the battery, maintaining stability and overall efficiency.
By keeping the ions on the move, the electrolyte contributes significantly to the battery’s charging capabilities and long-term performance.
The Process Of Discharging
Lithium ion batteries discharge by releasing stored energy through a chemical reaction between the positive and negative electrodes. This process allows for the continuous flow of electrons to power devices like smartphones and electric vehicles.
Releasing Energy From The Battery
When it comes to the process of discharging a lithium-ion battery, the first step involves releasing energy from the battery. This is an essential part of understanding how these batteries work. Once fully charged, a lithium-ion battery holds a significant amount of electrical energy waiting to be utilized. As the battery is connected to a device, the stored electrical energy starts to flow out, providing power to the device. This release of energy from the battery is what enables our devices to function and perform various tasks seamlessly.
The Movement Of Lithium Ions
To understand how energy is released from a lithium-ion battery, it’s essential to comprehend the movement of lithium ions. These ions are the key players in the battery’s operation. As the battery discharges, lithium ions move from the negative electrode. (also known as the anode) to the positive electrode (known as the cathode).
This movement occurs through a conductive electrolyte solution, which allows the lithium ions to flow freely and create a balanced electrical circuit. The movement of these lithium ions is precisely controlled by the internal structure of the battery, allowing for a consistent and controlled flow of energy.
Generating Electricity
As the lithium ions move from the anode to the cathode during the discharging process, they create a difference in electrical potential between the two electrodes. This voltage is another name for this potential difference, which is what ultimately powers our devices.
When a device is connected to the lithium-ion battery, the difference in electrical potential is harnessed and converted into usable electricity. This conversion process occurs through the flow of electrons in the external circuit, creating a continuous electrical current. This generated electricity is then utilized by the device, enabling it to operate efficiently.
In summary, the process of discharging a lithium-ion battery involves releasing energy stored within the battery, allowing it to flow out and power our devices. This energy release occurs as the lithium ions move from the anode to the cathode through a conductive electrolyte solution.
These ions’ motion results in an electrical potential difference, which, when harnessed, generates usable electricity. Understanding this process is crucial for optimizing the Lithium-ion battery lifetime and performance in various devices and applications.

Credit: www.bnl.gov
Advantages And Limitations Of Lithium Ion Batteries
Lithium-ion batteries have become the preferred choice for powering various devices, from smartphones to electric vehicles. Understanding the advantages and limitations of these batteries is crucial in realizing their full potential. In In this part, we will examine three important:
High Energy Density
Lithium-ion batteries are known for their impressive energy density, which speaks about the quantity of energy stored in a given volume or weight. These batteries have a far higher energy density than other types of rechargeable battery technologies, such as nickel-cadmium or lead-acid batteries.
This means that Lithium-ion batteries can store more energy in a lighter, more diminutive form, making them ideal for portable electronic devices where space and weight are critical considerations. Additionally, their high energy density allows for longer operating times, ensuring that your devices last longer between charges.
Long Lifespan
Another notable advantage of lithium-ion batteries is their long lifespan. With proper care, these batteries can endure hundreds, if not thousands, of charge and discharge cycles before their performance begins to degrade. This longevity makes them a cost-effective choice in the long run.
Compared to other rechargeable batteries that need regular replacements, lithium-ion batteries can save you both time and money. Moreover, They maintain their charge longer when not in use because of their low self-discharge rate, ensuring they are ready to power up your devices whenever needed.
Environmental Impact
When it comes to the effects on the environment, lithium-ion batteries have both advantages and limitations. On the positive side, these batteries do not contain hazardous materials like lead or mercury, minimizing their potential harm to the environment when it comes to disposal.
Additionally, the energy efficiency of lithium-ion batteries is higher compared to other battery types, reducing the overall carbon footprint of electronic devices using them.
However, it’s important to note that the production and disposal of lithium-ion batteries still have environmental implications. Safe and responsible recycling practices are crucial to minimize any negative environmental impact.
In summary, lithium-ion batteries offer several notable benefits, such as improved energy density, long lifespan, and reduced environmental impact, making them the preferred choice for a wide range of applications.
Understanding their limitations and adopting responsible usage and disposal practices will further enhance their overall benefits.
How Do Lithium-Ion Batteries Work Chemistry
Lithium-ion batteries work based on the between the positive and negative electrodes, the flow of lithium ions within the battery cell. The essential components of a lithium-ion battery include an Anode (negative electrode), a cathode (positive electrode), an electrolyte, and a separator.
Here’s a simplified explanation of the chemistry involved in the operation of a lithium-ion battery:
- Anode (Negative Electrode): Typically made of graphite, the anode is where lithium ions are stored when the battery is in a discharged state. During discharging (when the battery is providing power), lithium ions are released from the anode and move towards the cathode.
- Discharge reaction:
- Anode: LiC6→Li++e−+C6Anode: LiC6→Li++e−+C6
- Cathode (Positive Electrode): Usually composed of lithium cobalt oxide (LiCoO2) or similar materials, the cathode is where lithium ions are stored as soon as the battery is charged. Lithium ions charge during charging and move from the anode to the cathode.
- Charge reaction:
- Cathode: Li++e−+LiCoO2→Li2CoO2Cathode: Li++e−+LiCoO2→Li2CoO2
- Electrolyte: The electrolyte is a conductive medium that allows For the transfer of lithium ions between the anode and cathode. It is typically a lithium salt dissolved in a solvent.
- Separator: The separator is a porous material that physically separates the anode and cathode, preventing direct contact between them while allowing the flow of lithium ions.
When the battery is discharging (providing power):
- Lithium ions move from the anode through the electrolyte to the cathode.
- Electrons are released at the anode, creating an electric current that can be used to power devices.
- At the cathode, lithium ions and electrons combine, reducing the cathode material.
When the battery is charging:
- Lithium ions move from the cathode back to the anode.
- Electrons are supplied at the anode, and lithium ions are inserted into the anode material.
- The cathode material is oxidized as it releases lithium ions.
This process is reversible, allowing lithium-ion batteries to be charged and discharged multiple times. The critical advantage of lithium-ion batteries lies in their high energy density and relatively lightweight, making them popular for various applications, including portable electronic devices and electric vehicles.
Frequently Asked Questions For How Does A Lithium Ion Battery Work
How Does A Lithium-ion Battery Work Briefly?
A lithium-ion battery works by using lithium ions to move from the negative electrode to the positive electrode during discharge, and vice versa during charging. This movement creates an electric current that powers devices like smartphones and electric vehicles.
How Does A Lithium-ion Battery Recharge?
A lithium-ion battery works by using lithium ions to transition from the negative electrode to the positive electrode during discharge and vice versa during charging. This movement creates an electric current that powers gadgets such as electric cars and cell phones.
What Are The Disadvantages Of Lithium-ion Batteries?
Lithium-ion batteries have a few disadvantages. They can be expensive, prone to overheating, and have limited lifespan. They also degrade when exposed to extreme temperatures and can be hazardous if not disposed of properly. However, research efforts are ongoing to overcome these drawbacks and improve their performance.
How Is A Lithium Battery Different From A Regular Battery?
A lithium-ion battery recharges when electrical current flows back into it, reversing the discharge-related chemical reactions. This process restores the battery’s energy storage and allows it to be used again. Recharging typically involves connecting the battery to a power source, such as a charger or an electrical outlet.
Conclusion
To summarize, a lithium-ion battery is a marvel of modern technology. Its key components – the anode, cathode, and electrolyte – work together seamlessly to generate a steady flow of electric current. This portable and rechargeable power source has revolutionized various industries, from electronics to electric vehicles.
By understanding the workings of lithium-ion batteries, we can appreciate their importance and continue to drive advancements in energy storage technology. Let’s stay charged and keep innovating!

I am a technology Specialized writer and blogger based in the USA & UK. I have four years of experience in Technology, Social Media and all types of Battery’s like Solar Battery,Car Battery,Lithium Battery etc. So I work on solving these issues and give various tips on these issues.